OrganOx and eGenesis Forge Exclusive Clinical Co-Development Agreement to Advance Porcine-Supported Liver Rescue
- Orsco Lifesciences AG

- Nov 12, 2024
- 1 min read
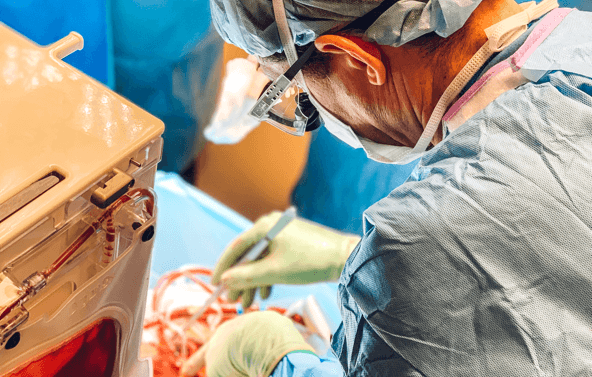
Oxford, UK & Cambridge, MA — November 12, 2024. OrganOx and eGenesis have entered into an exclusive clinical co-development agreement to co-create a novel liver support system pairing OrganOx’s NMP-based extracorporeal liver cross-circulation (ELC) platform with eGenesis’s human-compatible, gene-edited porcine liver (EGEN-5784). The goal: enhance survival and recovery in acute or acute-on-chronic liver failure. (OrganOx)
This collaboration targets an annual U.S. patient population of approximately 35,000 individuals suffering from liver failure—conditions with death rates as high as 50%. Early proof-of-concept ran three trials under the PERFUSE‑2 study, evaluating feasibility of supporting human patients with EGEN‑5784 integrated via OrganOx’s ELC system. OrganOx and eGenesis plan to submit a U.S. IND application in 2025 to launch a Phase 1 trial. (OrganOx)
Governance note: Ørn R. Stuge, MD, MBA serves as Executive Chairman at OrganOx. He brings proven medtech leadership and strategic board experience to support platform innovations like this one.




Comments